Let's try something a little more complicated...
You are observing a mystery radioactive isotope. At 4 pm, there are 2.5 grams and at 9 pm, there are 1.7 grams. What's the half-life?
We'll have to solve for h this time!
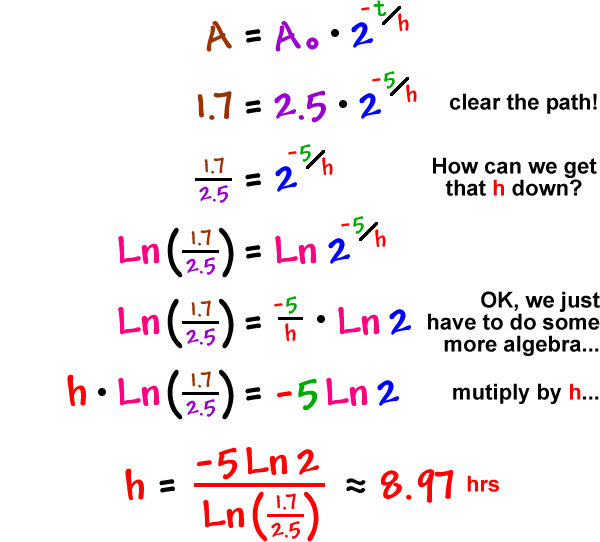
The half-life is about 8.97 hrs.
TRY IT:
Doing some digging on an alien planet, you find the bones of a Carbon-based alien life form. If a live alien specimen has 0.7 mg of Carbon-14 in it's bones and you measure 0.2 mg, how old is the specimen?